Science - Question Paper (2014)
Class 10 Science Question Paper (SA1 - September 2014)
Time Allowed: 3 Hours Maximum Marks: 90
General Instructions:
(i) The Question Paper comprises of two sections, A and B. Attempt both sections.
(ii) All questions are compulsory
(iii) All questions of Section A and all questions of Section B are to be attempted separately.
(iv) Question numbers 1 to 3 in Section A carry one mark each. These questions are to be answered in one word or in one sentence.
(v) Question numbers 4 to 6 in Section A are three marks questions. Answer each in about 30 words each.
(vi) Question Numbers 7 to 18 in Section A are three marks each. Answer each question not more than 50 words.
(vii) Questions 19 to 24 in section A are five marks each. Answer each question in not more than 70 words.
(viii) Questions numbers 25 to 33 in section B are multiple choice questions based on practical skills. Each question carries one mark.Select the most appropriate choice from the given choices.
(ix) Question Numbers 34 to 36 in Section B are questions based on practical skills and are two marks.
(i) The Question Paper comprises of two sections, A and B. Attempt both sections.
(ii) All questions are compulsory
(iii) All questions of Section A and all questions of Section B are to be attempted separately.
(iv) Question numbers 1 to 3 in Section A carry one mark each. These questions are to be answered in one word or in one sentence.
(v) Question numbers 4 to 6 in Section A are three marks questions. Answer each in about 30 words each.
(vi) Question Numbers 7 to 18 in Section A are three marks each. Answer each question not more than 50 words.
(vii) Questions 19 to 24 in section A are five marks each. Answer each question in not more than 70 words.
(viii) Questions numbers 25 to 33 in section B are multiple choice questions based on practical skills. Each question carries one mark.Select the most appropriate choice from the given choices.
(ix) Question Numbers 34 to 36 in Section B are questions based on practical skills and are two marks.
| Q25: | Four students studied reactions of zinc and sodium carbonate with dilute hydrochloric acid and sodium hydroxide solutions. They presented their results as follows. The (✓) shows evolution of gas and (✗) shows no reaction. Which one of the following is right set? (a)
(b)
(c)
(d)
| 1 | ||||||||||||||||||||||||||||||||||||
| Q26: | Dilute NaOH solution and solid sodium carbonate: (a) react only on heating (b) react very slowly (c) do not react (d) react vigrously | 1 | ||||||||||||||||||||||||||||||||||||
| Q27: | The colour of Cu metal is (a) reddish brown (b) blue (c) green (d) grey | 1 | ||||||||||||||||||||||||||||||||||||
| Q28: | Suresh was asked to carry out a displacement reaction which would show the following: (i) Formation of colourless solution. (ii) Black deposits The reactants he should use are: (a) Fe(s) and Al2(SO4)3 (aq) (b) Al(s) and FeSO4 (aq) (c) Zn(s) and CuSO4 (aq) (d) Fe(s) and ZnSO4 (aq) | 1 | ||||||||||||||||||||||||||||||||||||
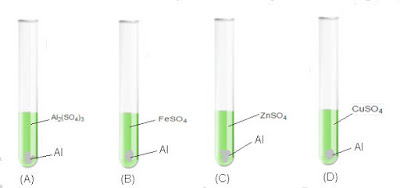
| Q29: | Monica was experimenting of comparing reactivity of metals in the laboratory. She was given aluminium metal and was asked to check reactivity by using four solutions as show below. She would observe that the reaction takes place in: (a) A and B (b) B, C and D (c) A, C and D (d) C and D | 1 | ||||||||||||||||||||||||||||||||||||
| Q30: | In an experiment to find the equivalent resitance of a series combination of two resistors of 3Ω and 4Ω in the circuit diagram given. The circuit will give: (a) Incorrect reading for current I and correct reading for voltage V (b) Incorrect readings for both current I and voltage V (c) Correct reading for current I and incorrect reading for voltage V (d) Correct readings for voltage V and current I | 1 | ||||||||||||||||||||||||||||||||||||
| Q31: | A student joined three resitances as shown in the circuit below. The current recorded by ammeter (A) is: (a) 0.25 A (b) 0.5 A (c) 0.75 A (d) 1 A | 1 | ||||||||||||||||||||||||||||||||||||
| Q32: | The iodine solution is: (a) Pure iodine dissolved in water (b) Potassium iodide in water (c) Iodine dissolved in potassium iodide (d) Potassium iodide dissolved in iodide | 1 | ||||||||||||||||||||||||||||||||||||
| Q33: | See the figure below and choose the correct set-up to demonstrate that CO2 is given out during respiration: (a) A (b) B (c) C (d) D | 1 | ||||||||||||||||||||||||||||||||||||
| Q34: | An iron nail is dipped in the solution of copper sulphate for about 30 minutes, state the change in colour observed. Give the reason for the change. | 2 | ||||||||||||||||||||||||||||||||||||
| Q35: | A student verifying Ohm's law computed the value of resistance of a resistor for each set of observation. However the values of the resistor were slightly different from the actual value. Is his experiment wrong? Justify your answer. | 2 | ||||||||||||||||||||||||||||||||||||
| Q36: | Draw a labelled diagram of stomatal apparatus with closed stomatal pore. Label its different parts and show the directions of water flowing. | 2 | ||||||||||||||||||||||||||||||||||||





No comments:
Post a Comment
We love to hear your thoughts about this post!
Note: only a member of this blog may post a comment.